Abstract
Chimeric antigen receptor (CAR) therapies are potent and powerful therapeutics shown to successfully mount immune responses against hematologic malignancies. While effective, CAR therapies are often associated with adverse toxicities that limit their safety including life-threatening side effects as seen in anti-ERBB2 CAR (Morgan et al. 2010.). These toxicities stem from on-target, off-tumor activity due to CAR-mediated targeting of tumor-associated antigens expressed on both tumor and healthy cells. Additionally, high avidity format therapies, including CAR-T, transgenic T cell receptor (TCR), and TCR-like CAR therapies, can lead to unpredictable or lethal toxicity as seen in anti-CD19 CAR and anti-ERBB2 CAR. To address this, we have developed a unique approach to tune CAR expression into a safe and efficacious range, thereby increasing the therapeutic window. Our goal is to reduce or eliminate off-tumor toxicity while maintaining strong CAR-mediated on-tumor function. This approach attempts to regulate CAR activity by controlling its expression level via sequence-directed codon engineering. Overall, we hypothesize that this method to reduce surface expression of CARs will reduce off-tumor toxicity and maintain strong on-tumor activity.
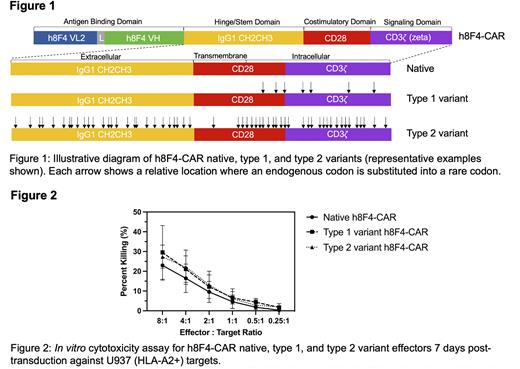
Sequence-directed expression control to tune CAR activity is achieved by strategically substituting specific codons with their synonymous rare isoforms within the non-antigen binding domains of the CAR construct. This technique utilizes inherent codon bias present within the human genome where specific codons are expressed at different frequencies compared to others. Using the rarest, or least frequently used, codons in the human genome, we created multiple variants of a TCR-like CAR developed by our group named h8F4-CAR, which targets the PR1/HLA-A2 peptide present in myeloid leukemia (Ma et al. 2016.). Variants contained rare codon substitutions across non-antigen binding domains using strategic engineering of parameters including codon location, density, distribution, and prevalence. Simplified, variants were created in 2 subtypes (Figure 1): intracellular-focused rare codon substitutions (type 1) and combined extracellular and intracellular rare codon substitutions (type 2). Type 1 variants contained between 6 and 30 rare codons while type 2 variants contained between 27 and 57 rare codons. Following development, each variant was monitored over a 28-day period in the Jurkat mutant line, J.RT3-T3.5, to assess changes in surface expression. Every 7 days, h8F4-CAR expression was verified via flow cytometry and surface expression was determined by median fluorescence intensity (MFI). Results revealed that type 2 variants containing higher numbers of rare codons displayed lower surface protein expression (by MFI) compared to native h8F4-CAR by day 28 (between 1 to 1.5-fold lower), thus consistent with findings in yeast (Hoekema et al. 1987.). To evaluate overall CAR protein expression, anti-CAR immunoblotting of whole cell lysates was performed. In addition to full length native h8F4-CAR (73kDa), novel, truncated isoforms were identified (45kDa and 60kDa in type 1 variants and 30kDa and 45kDa in type 2 variants). This suggests inefficient protein processing may reduce surface expression via altered translation caused by the rare codon substitutions. This is conceivable from codon bias originating evolutionarily as certain (preferred) codons may play a more robust role in translational efficiency, which can be exploited to better control CAR protein expression levels. To further evaluate the impact that changes to CAR expression have on activity, we performed functional cytotoxicity experiments via standard calcein-AM in vitro cytotoxicity assays using human T cells bearing each variant against leukemia targets. Results showed each variant maintained strong cytotoxic capacity against leukemia targets (i.e., U937 HLA-A2+) (Figure 2), and studies are currently ongoing to determine if these variants show lower activity against non-leukemia targets. Altogether, these results suggest that codon-mediated, sequence-directed expression control of CAR is possible and demonstrates a novel approach to modulate CAR expression to potentially reduce off-tumor toxicity while preserving potent on-tumor killing to achieve safe and efficacious therapeutic responses.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal